Scientists from China have investigated how short peptide chains aggregate together in order to deepen our understanding of the process which is crucial for drug stability and material development.
Their study, published in JACS Au, provides valuable insights into how short proteins called peptides interact, fold, and function. These findings have significant implications for medicine, material science, and biotechnology.
Peptides are short chains of amino acids that play essential roles in the body by building structures, speeding up chemical reactions, and supporting our immune system. The specific function of a protein is determined by how its amino acids interact with each other and aggregate into a three-dimensional structure.
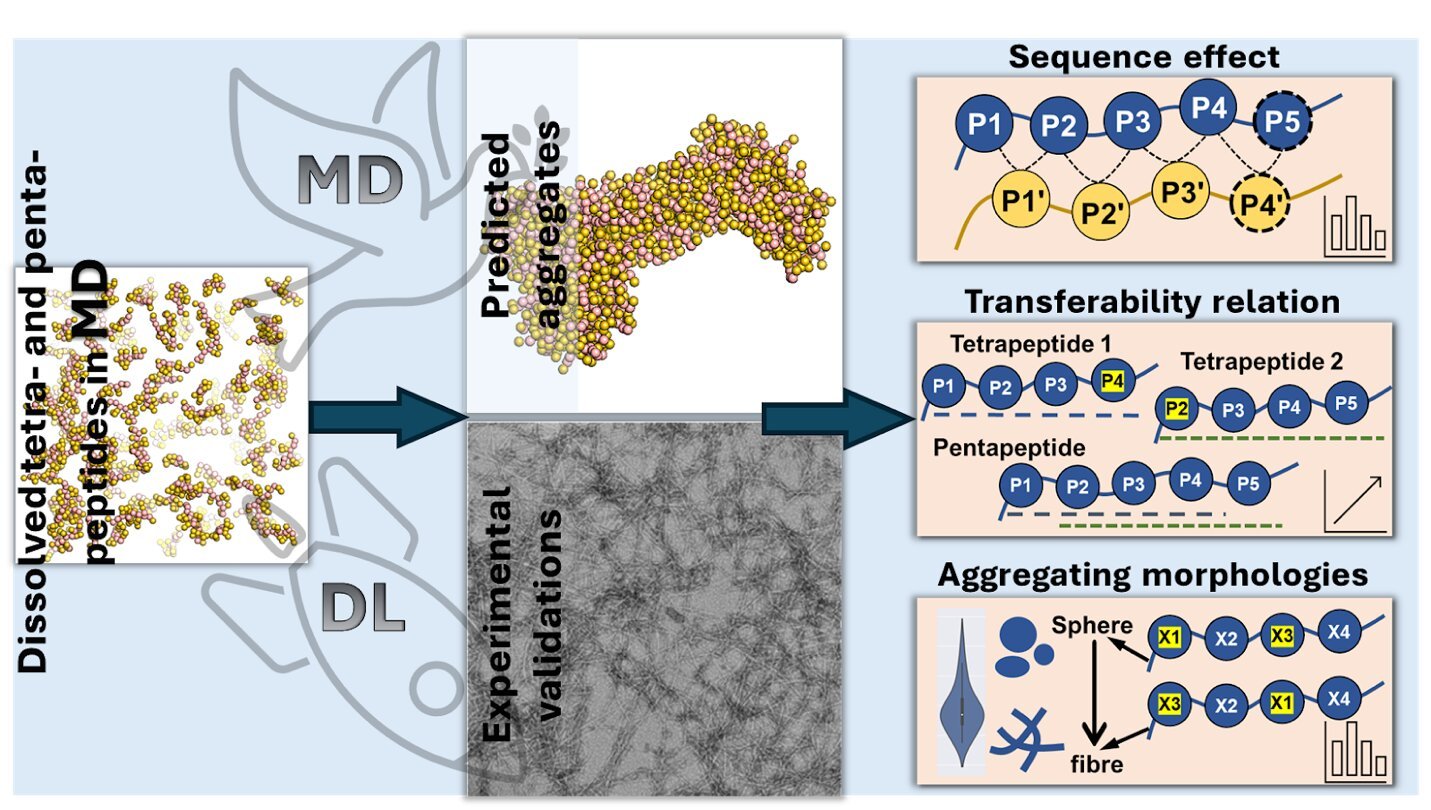
The research team used molecular dynamics simulations together with advanced AI techniques, including deep learning models like Transformer Regression Networks, to predict how various peptides of four or five amino acids (tetrapeptides and pentapeptides, respectively) would aggregate based on their amino acid sequence.
By analyzing 160,000 tetrapeptides and 3.2 million pentapeptides, they discovered that certain amino acids, particularly aromatic ones like tryptophan, phenylalanine, and tyrosine, significantly enhance aggregation, especially when located towards one end (the C-terminus) of the peptide chain.
This is probably because aromatic amino acids have ring-shaped structures that attract each other through their electron clouds, normally termed as “π-π” interactions, which helps them clump together. By contrast, hydrophilic amino acids, such as aspartic acid and glutamic acid, inhibit aggregation due to the strong interaction with water molecules that prevents the peptides from sticking together.
The study also showed that changing the amino acid sequence affects aggregation. For example, adding aromatic amino acids to the end of the peptide chain increases aggregation, while placing negatively charged amino acids at the beginning reduces it. The team also found that peptides clump together into different shapes based on the types and positions of their amino acids.
“Amino acids with a charge generally cause peptides to form long, thread-like structures, while those that avoid water tend to create round, ball-like clusters,” explains Dr. Wenbin Li, an assistant professor at Westlake University and corresponding author of the study.
“We also discovered that by understanding how tetrapeptides stick to each other, we can predict how pentapeptides will behave, making it easier to predict how longer peptides will clump together.”
The findings provide important guidelines for predicting and managing how peptides aggregate.
“This knowledge could help in creating new materials, designing more stable drugs and drug delivery systems, and understanding diseases linked to peptide aggregation, such as Alzheimer’s disease, where clumped amyloid-beta peptides form damaging plaques in the brain,” says Dr. Jiaqi Wang, an assistant professor at Xi’an Jiaotong-Liverpool University (XJTLU) and first author of the study.
“It can also improve biotechnology, such as semiconductors, biosensors and diagnostics, ensuring these tools work accurately and consistently.
“By offering new insights into peptide aggregation, this research is set to advance biochemistry, materials science, and computational biology. It also demonstrates the integration of AI into scientific discovery. These advances could lead to breakthroughs in medical treatments, eco-friendly products, and innovative technologies.”
More information:
Jiaqi Wang, Zihan Liu, Shuang Zhao, Yu Zhang, Tengyan Xu, Stan Z. Li, Wenbin Li. Aggregation Rules of Short Peptides JACS Au (2024). DOI: 10.1021/jacsau.4c00501. pubs.acs.org/doi/10.1021/jacsau.4c00501
Provided by
Xi’an jiaotong-Liverpool University
Citation:
Exploring peptide clumping for improved drug and material solutions (2024, September 3)
retrieved 3 September 2024
from https://phys.org/news/2024-09-exploring-peptide-clumping-drug-material.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.