Researchers from Kyushu University have revealed that a zeolite material called Na-ZSM-5 is effective in improving the chemical conversion of biomass into olefins—a precursor chemical that makes everything from plastics to pharmaceuticals—using microwaves.
Publishing their work in Chemical Engineering Journal, the team explains that microwave heating of Na-ZSM-5 could open doors to a more energy-efficient and sustainable chemical industry.
If you want to synthesize complex organic compounds, whether it be plastics, pharmaceuticals, or food additives, you generally need to start with chemical precursors with simple structures. Naturally, finding ways to efficiently and sustainably synthesize precursor chemicals is an extensively researched field.
A commonly used method of synthesizing these essential chemicals is via a process called reforming of naphtha. However, this process requires high amounts of energy and releases carbon dioxide. Cooking oil waste and microalgal oils have been considered as alternative and inexpensive sources to synthesize said simple chemicals.
These oils can be converted using a method called ‘catalytic cracking’ with the help of a material called zeolite. Zeolite is a porous natural material, commonly used as a catalyst or as an absorbent.
In the catalytic cracking process, materials need to be heated up to temperatures as high as 500–600°C. In addition to being very energy intensive, operating at such temperatures can cause a buildup of unwanted deposits, a phenomenon known as coking, which reduces the lifetime of the catalyst.
In the present study, Associate Professor Shuntaro Tsubaki from Kyushu University’s Faculty of Agriculture and his team experimented with microwaves to heat zeolite catalysts to the required temperature without the negative effects like coking.
“Microwaves interact directly with materials and can selectively deliver energy to them, enabling significant energy savings compared to conventional heat-convective processes,” explains Tsubaki. “In particular, microwaves can accelerate gas–solid catalysis by passing straight through the gas phase and selectively heating the solid catalyst. They achieve this by forming spatial hot spots inside the catalyst bed.”
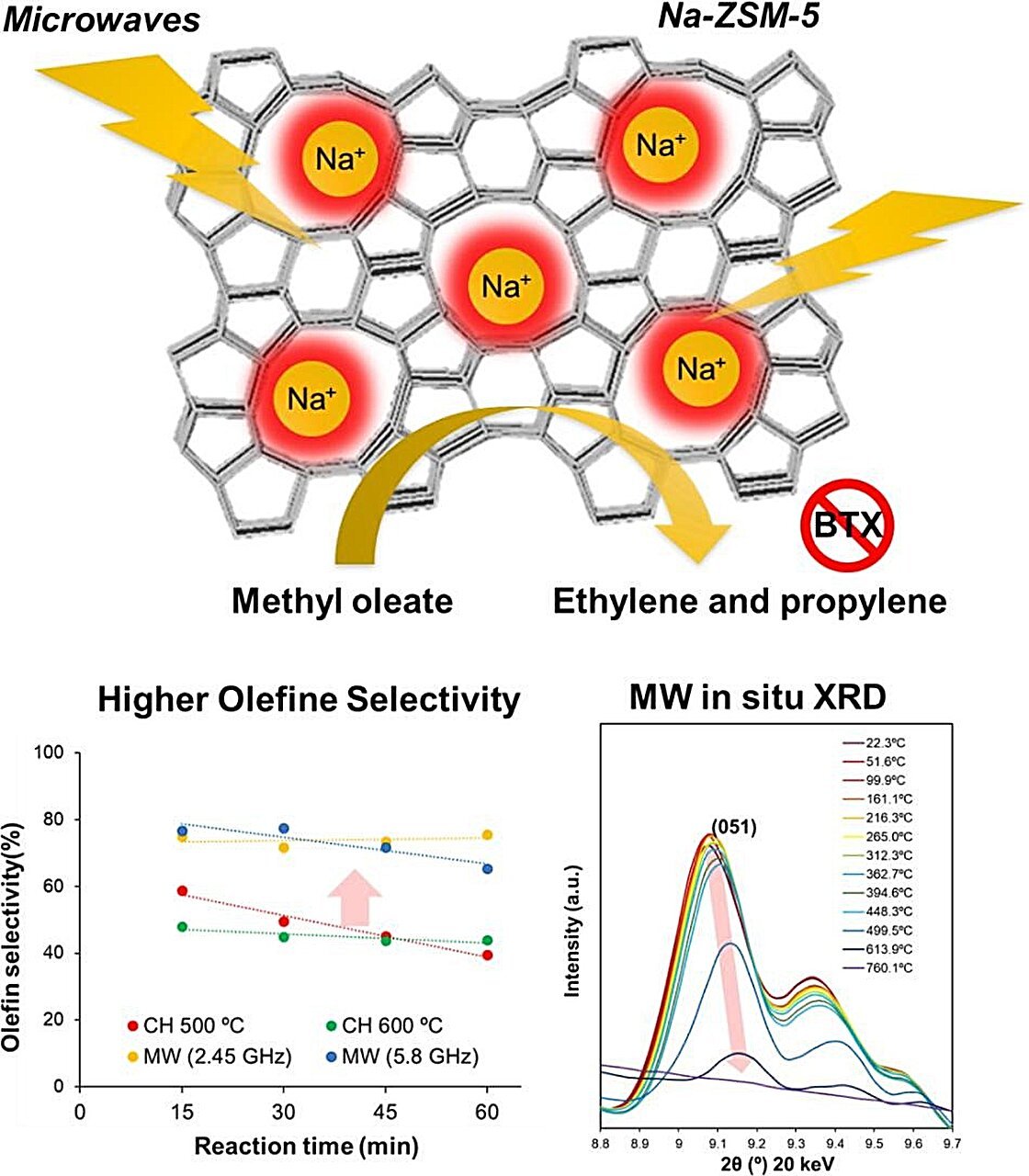
In their study, the researchers first compared various zeolite catalysts, looking for the ones that could be effectively heated by microwaves and deliver good catalytic performance. Using both theoretical and experimental analyses, they narrowed it down to Na-ZSM-5, a sodium ion substituted zeolite.
Next, to showcase the advantages of microwave heating versus conventional heating, the researchers performed a catalytic conversion of methyl oleate. When microwave heating was used, Na-ZSM-5 outperformed other catalysts, achieving a high conversion efficiency of fatty acid esters into olefins with high selectivity. Moreover, carbon dioxide production was suppressed to only 1.3% of the total reaction output, and carbon monoxide was not produced at all.
Most importantly, microwave heating of Na-ZSM-5 to 500°C resulted in four times higher olefin production compared to conventional heating to 500°C. This was in part caused by a higher selectivity of Na-ZSM-5 to produce olefins instead of other compounds. Moreover, coke formation was not observed when microwave heating was used, even at the high temperature of 600°C.
Finally, to shed light on why microwave heating improved upon various aspects of the catalytic process, the researchers analyzed the local structural changes in the zeolite when exposed to microwaves. Interestingly, they found that microwave absorption caused localized temperatures of over 1000°C in the zeolite’s crystal lattice, even as the temperature of the bulk material remained at 500°C. These extreme temperatures were likely responsible for driving the selective production of olefins.
Microwave heating of catalysts could significantly improve catalytic biomass conversion and help in achieving the sustainability goals of the modern chemical industry.
“Our findings are expected to contribute to the further electrification of the chemical industry. Since microwaves can be generated from renewable energy sources such as solar and wind, we can reduce the environmental impact of the synthesis of these fundamental chemicals,” says Tsubaki.
The researchers are planning on further enhancing microwave-driven catalytic processes, seeking to improve yield and energy efficiency while scaling up their capacity. They hope their efforts could lead to a new era in sustainable chemical manufacturing.
More information:
Shunsuke Ota et al, Microwave-enhanced catalytic conversion of fatty acid ester to olefins by Na-ZSM-5, Chemical Engineering Journal (2024). DOI: 10.1016/j.cej.2024.154737
Provided by
Kyushu University
Citation:
Zeolite catalyst method uses microwaves to convert waste cooking oil into useful chemicals (2024, September 9)
retrieved 9 September 2024
from https://phys.org/news/2024-09-zeolite-catalyst-method-microwaves-cooking.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.