Biodiesel, a green alternative to conventional diesel, has been shown to reduce carbon dioxide emissions by up to 74%. Biodiesel is produced through transesterification, converting triglycerides into biodiesel and producing glycerol as a low-value byproduct.
Since glycerol makes up about 10% of the output, efforts have focused on boosting its value. One method involves electrochemical oxidation, turning glycerol into high-value three-carbon compounds like dihydroxyacetone (DHA) and glyceraldehyde (GLYD), though past approaches often yielded unstable or low-value products under strong alkaline conditions.
In a study published in the Journal of Catalysis on 15 August 2024, researchers led by Associate Professor Tomohiro Hayashi from Tokyo Institute of Technology (Tokyo Tech) and Professor Chia-Ying Chiang from National Taiwan University of Science and Technology, Taiwan, have developed a highly selective and efficient glycerol electrooxidation (GEOR) process that can lead to the production of valuable 3-carbon (3C) products.
“Establishing an electrochemical route for a highly selective and efficient glycerol electrooxidation process to desirable 3C products is essential for biodiesel production,” say Hayashi and Chiang.
Selective oxidation of glycerol is challenging due to its structure. Glycerol has three –OH groups: two on primary carbon atoms and one on a secondary carbon atom. This arrangement creates steric hindrance, making it hard for reactants to target specific –OH groups for oxidation. In alkaline conditions, the –OH groups also cause unwanted side reactions that break carbon-carbon bonds, resulting in two-carbon or one-carbon compounds instead of the desired three-carbon products.
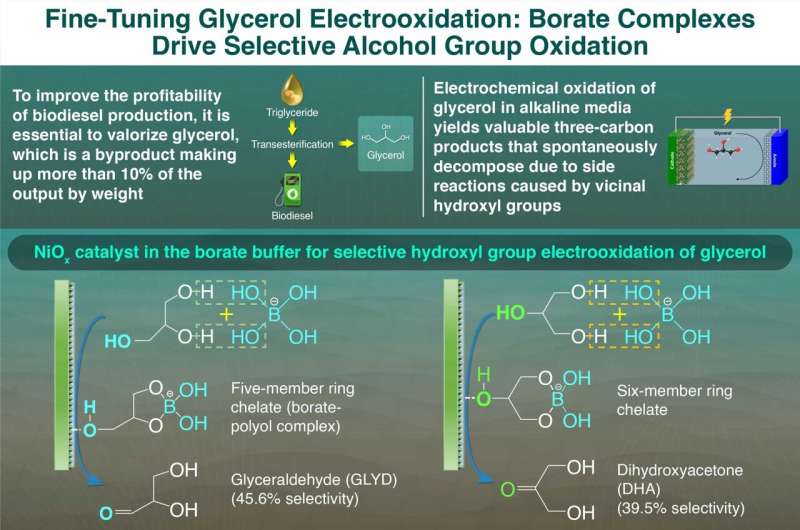
To address this, the researchers conducted GEOR using sodium borate and bicarbonate buffer as a mild alkaline electrolyte and a nickel-oxide (NiOx) catalyst. The sodium borate helps protect a certain –OH group, improving the selectivity of the reaction, while the NiOx catalyst enhances the efficiency of the electrooxidation process. Sodium borate forms coordination complexes with glycerol’s primary and secondary alcohol groups to form GLYD and DHA respectively.
However, the final product depends on the ratio of borate to glycerol. To understand how different concentrations of glycerol and borate affect the electrooxidation process, a fixed concentration of 0.1 M borate buffer was reacted with varying concentrations of glycerol (0.01, 1, 2.0 M) and a fixed concentration of 0.1 M glycerol with varying concentrations of borate buffer (0.01, 0.05, 0.10, and 0.15 M). while maintaining a pH of 9.2.
Higher borate concentrations were found to increase the selectivity for 3C products, particularly DHA, with the highest selectivity of up to 80% observed at a borate concentration of 0.15 M. This improvement is attributed to the increased buffer capacity provided by the borate solution, which helps maintain a stable pH during the reaction and stabilizes the borate-glycerol complex for further oxidation into 3C compounds.
Conversely, increasing the glycerol concentration reduced both the yield and selectivity of 3C products. At a glycerol concentration of 1 M, GLYD was the main product, with a selectivity of 51%.
The difference in the type of 3C product was found to be related to the formation of different glycerol-borate complexes. Using Raman spectroscopy, the researchers found higher borate concentrations favor six-membered ring complexes, promoting secondary –OH oxidation and DHA production. Conversely, higher glycerol concentrations favor five-membered ring complexes, leading to primary –OH oxidation and GLYD formation.
“Five-membered ring complexes were more likely to form in the electrolyte with a borate-to-glycerol ratio of 0.1, whereas six-membered ring complexes became more prominent in the electrolyte with a borate-to-glycerol ratio of 1.5,” say Hayashi and Chiang.
These findings present a promising strategy for transforming glycerol into valuable products, boosting the sustainability and profitability of biodiesel production.
More information:
Giang-Son Tran et al, Tuning selectivity toward three-carbon product of glycerol electrooxidation in borate buffer through manipulating borate/glycerol molar ratio, Journal of Catalysis (2024). DOI: 10.1016/j.jcat.2024.115715
Provided by
Tokyo Institute of Technology
Citation:
Boosting glycerol’s value: A new process makes biodiesel more profitable (2024, September 3)
retrieved 3 September 2024
from https://phys.org/news/2024-09-boosting-glycerol-biodiesel-profitable.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
