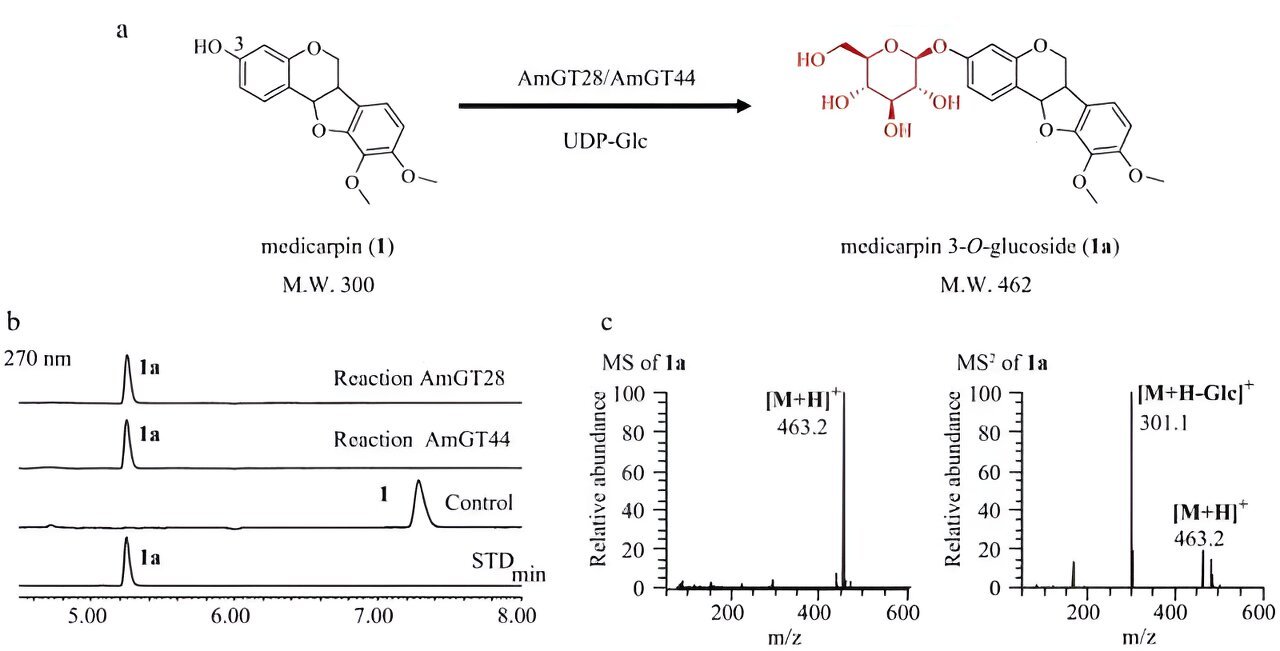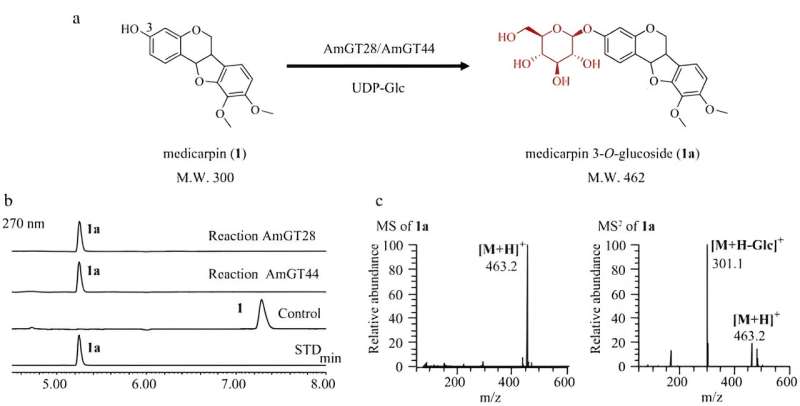
A research team has identified two highly efficient glycosyltransferases, AmGT28 and AmGT44, from Astragalus membranaceus, which convert medicarpin to medicarpin 3-O-glucoside and show a preference for pterocarpans over isoflavonoids.
These enzymes enable the biosynthesis of glycosides and have been employed in a whole-cell biocatalytic system with high conversion rates. This discovery offers valuable catalytic tools for the production of biologically active pterocarpan glycosides, which have potential applications in pharmaceuticals and biotechnology.
Astragalus root, derived from Astragalus membranaceus, is widely utilized in herbal medicine and dietary supplements due to its rich content of bioactive pterocarpan glycosides.
Glycosylation, mediated by UDP-glycosyltransferases (GTs), is essential in the biosynthesis of these compounds. While flavonoid GTs have been well-studied, specific GTs for pterocarpans remain poorly understood , with existing ones showing low selectivity and conversion rates.
A study published in Medicinal Plant Biology on 5 June 2024 aims to address this gap by identifying two efficient pterocarpan GTs, AmGT28 and AmGT44, and establishing a whole-cell catalytic system for pterocarpan glycosylation.
The research team conducted molecular cloning and functional characterization of two glycosyltransferases, AmGT28 and AmGT44, from A. membranaceus.
The purified recombinant proteins of AmGT28 and AmGT44 showed an almost 100% conversion rate in catalyzing medicarpin to medicarpin 3-O-glucoside, as confirmed via LC/MS analysis.
Furthermore, to assess substrate promiscuity, various aglycones from Astragalus root and derivatives were tested. Both enzymes exhibited high conversion rates for pterocarpans and isoflavonoids, with a new product 7-O-glucoside 5a being identified.
AmGT44 exhibited superior conversion rates for specific substrates compared to AmGT28, which was influenced by substrate binding modes. Sugar donor preference was also evaluated, showing that AmGT44 could use UDP-Glc, UDP-Xyl, and UDP-GlcNAc, whereas AmGT28 exhibited limited versatility.
Moreover, the optimal reaction conditions for AmGT44 were established, revealing a preference for pterocarpans and a whole-cell biocatalytic system was developed to simplify enzyme usage, thereby achieving high conversion rates for pterocarpans, especially with AmGT44, which reached up to 100% conversion and 78.66 μg/mL titer in scaled-up reactions.
However, lower conversion rates for some polar compounds indicated the need for further optimization.
According to the study’s senior researcher, Xue Qiao, “This study has provided efficient catalytic tools for the biosynthesis of pterocarpan glycosides.”
In summary, the study identified two glycosyltransferases, AmGT28 and AmGT44, from A. membranaceus, which were found to efficiently catalyze the conversion of medicarpin to medicarpin 3-O-glucoside, with a preference for pterocarpans over isoflavonoids.
A whole-cell biocatalytic system was established, achieving up to 100% conversion rates and high titers.
The findings of this research offer valuable tools for biosynthesizing bioactive pterocarpan glycosides, with potential applications in pharmaceuticals and biotechnology. Future work could optimize and expand these systems for industrial-scale production.
More information:
Bai-Han Gao et al, Characterization of two pterocarpan glycosyltransferases in Astragalus membranaceus and their application in whole-cell biocatalysis, Medicinal Plant Biology (2024). DOI: 10.48130/mpb-0024-0013
Provided by
Chinese Academy of Sciences
Citation:
Efficient glycosyltransferases from A. membranaceus enable biosynthesis of bioactive pterocarpan glycosides (2024, August 12)
retrieved 12 August 2024
from https://phys.org/news/2024-08-efficient-glycosyltransferases-membranaceus-enable-biosynthesis.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.