Scientists from Tokyo Metropolitan University have identified a new photoreceptor in cyanobacteria with a modification in part of its structure which makes it sensitive to green/teal light. The photoreceptor belongs to a family usually sensitive to red/green light in the environment.
They identified the parts of its amino acid structure responsible for this behavior; editing them helped restore sensitivity to red and green light, a remarkable example of molecular “plasticity” in action.
Cyanobacteria, also known as blue-green algae, are phenomenally important players in shaping the natural world as we know it. They are the first organisms on Earth known to have produced oxygen, using light to break down water molecules.
The same chemical process is a distant relative of photosynthesis seen in plants. The chemical apparatus that helps such processes in cyanobacteria are known as cyanobacteriochromes (CBCRs), cyanobacteria-specific photoreceptors; these are amino acid-based constructs which make them sensitive not only to the amount, but the color of light in the surroundings, helping them acclimate to convert energy as efficiently as possible.
But how exactly do cyanobacteria tell apart color? In the case of CBCRs, they bind a chemical containing a chemical group known as a linear tetrapyrrole. There is a vast range of linear tetrapyrrole pigments, with a color response determined by the length of the cloud of “conjugated” electrons running along.
For example, phycocyanobilin (PCB), a blue pigment, will be bound by a CBCR and help it respond to red or green light, switching reversibly between two different absorbing states depending on the ratio of red and green light in the environment.
On the other hand, CBCR binding phycoviolobilin (PVB), a pink pigment, will respond to violet or yellow light in a similar way. Interestingly, PCB and PVB are an “isomer” pair, in that they share the same chemical composition, but with double bonds at different positions, which drastically switches their color.
The way in which pigments bind to CBCRs is remarkably sensitive to its specific structure, as determined by its amino acid sequence, but the ways in which different pigment/CBCR pairs give rise to a whole myriad of responses to light is not yet fully understood.
Now, a team of researchers led by Associate Professor Rei Narikawa from Tokyo Metropolitan University have uncovered another piece of the puzzle, discovering a new CBCR from the family of photoreceptors which should be sensitive to red and green light. The paper is published in the journal Protein Science.
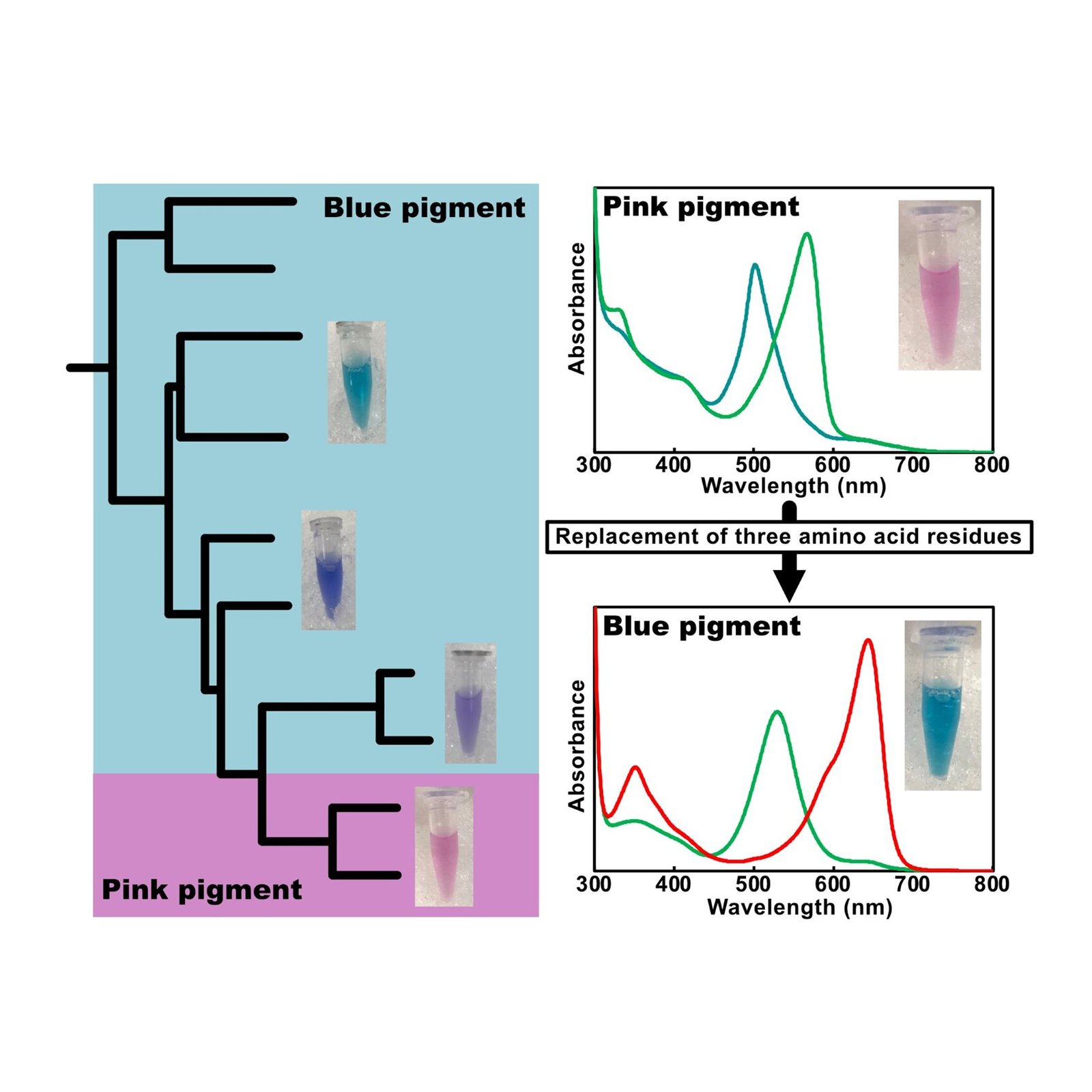
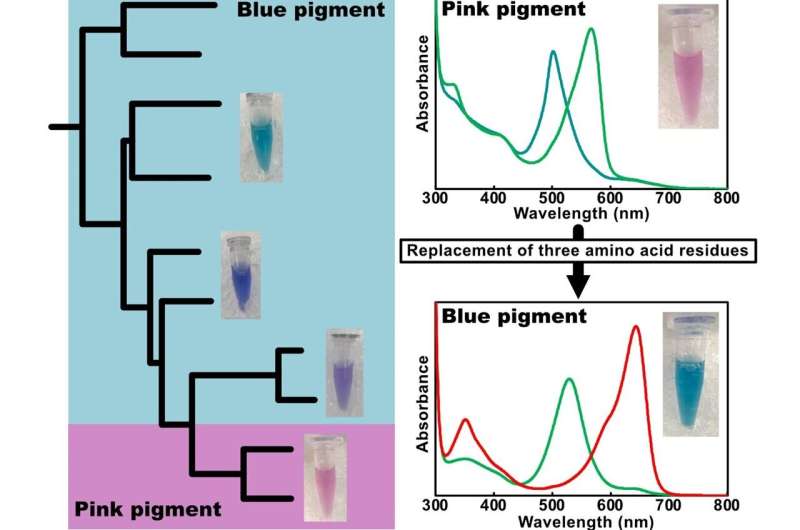
Uniquely, this CBCR binds PVB, the pink pigment, and responds to green or teal light. Given the differences with normal PVB binding CBCRs, the team concluded that the pigment was bound to the CBCR in a new, different way.
By analyzing the structure carefully, they also identified three key amino acid residues which they determined to be key to this unusual response. Having modified them, the newly edited CBCR could induce the PVB to become PCB again (the blue pigment), restoring sensitivity to red and green light.
The work highlights the remarkable diversity and “plasticity” of CBCRs, the ability to be molded or changed, expanding our understanding of how cyanobacteria can “see” the world in color.
More information:
Hiroki Hoshino et al, Red/green cyanobacteriochromes acquire isomerization from phycocyanobilin to phycoviolobilin, Protein Science (2024). DOI: 10.1002/pro.5132
Provided by
Tokyo Metropolitan University
Citation:
Novel photoreceptor sheds light on how cyanobacteria ‘see’ color (2024, August 19)
retrieved 19 August 2024
from https://phys.org/news/2024-08-photoreceptor-cyanobacteria.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.