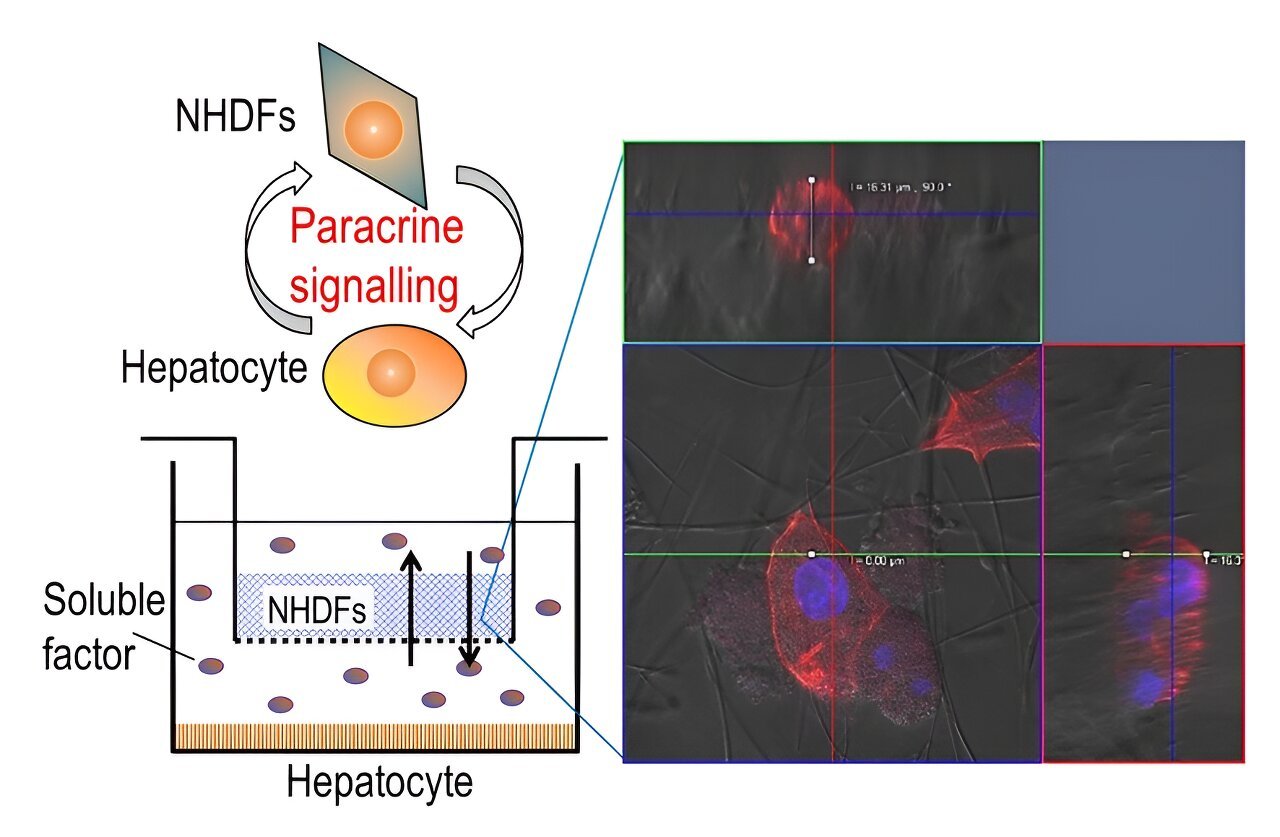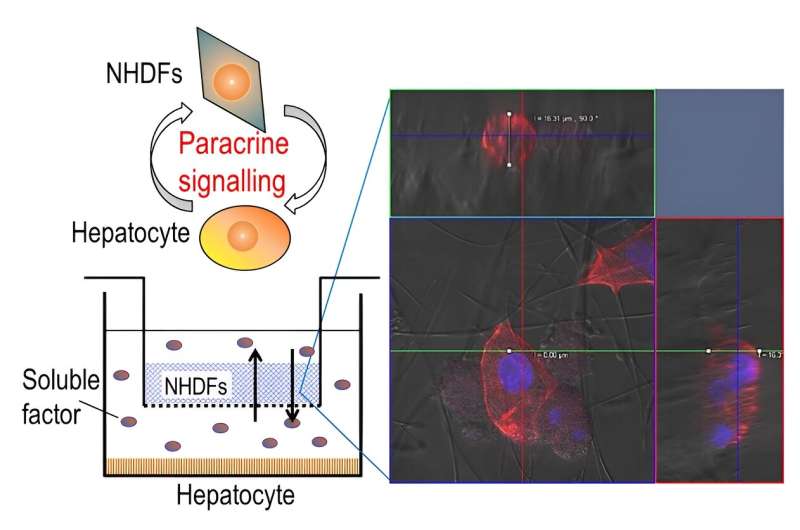
Communication and coordination among different cells are fundamental aspects that regulate many functions in our body. This process, known as paracrine signaling, involves the release of signaling molecules by a cell into its extracellular matrix (ECM) or surroundings to communicate changes in its cellular processes or the local environment. These signaling molecules are then detected by neighboring cells, leading to various cellular responses.
For instance, during cell/tissue injury, the paracrine signaling process releases growth factors that signal nearby stem cells to assist in tissue repair in the form of scar tissue formation or blood clotting. Similar processes occur in the regulation of other vital functions, such as digestion, respiration, and reproduction. Additionally, paracrine signals influence the expression and activity of enzymes involved in drug metabolism and play a role in drug–drug interactions.
The signaling molecules, which may contain proteins and genetic material, are transported within tiny vesicles called exosomes. These vesicles serve as valuable biomarkers for various diseases and can even be engineered to carry drugs, making them a highly effective targeted drug delivery system. Notably, the hormone oxytocin and the neurotransmitter dopamine are paracrine messengers.
To investigate the impact of exosomes on cell–cell interactions, a team of researchers, including Professor Hidenori Otsuka and Dr. Shohei Ishikawa from Tokyo University of Science, has recently developed an innovative cell culture system. Their findings were published in the journal Biotechnology and Bioengineering on May 19, 2023.
The proposed system addresses several limitations associated with conventional co-culture systems, where different cell types are cultured in proximity.
“When cells are cultured three-dimensionally on a scaffold such as a normal hydrogel, the scaffold on the membrane insert separating them contracts as the cells proliferate and functionally differentiate, inhibiting the permeation of cell-secreted factors such as proteins and exosomes and resulting in a decline in functionality,” explains Prof. Otsuka. Another challenge in these cultures is to distinguish between the specific roles of cell-to-cell contact via direct physical interactions and paracrine signaling.
To tackle these challenges, the research team designed a three-dimensional segregated co-culture model with a well plate—rectangular plate with many separated wells—consisting of two cell types separated by a membrane insert with silica nonwoven fabric (SNF), an interconnected network of highly porous electrospun fabric. In such a segregated system, cell-to-cell interactions via direct contact are eliminated.
In addition, the high mechanical strength and interconnected porous network of SNF can facilitate cell growth without undergoing shrinkage or causing a decline in cell function. This allows interactions between different cell types to be maintained and studied over extended periods.
Using the proposed co-culture model, the researchers cultured primary hepatocytes from rats and normal human dermal fibroblasts in separate compartments but within the same culture environment. These two cell types are found within the liver tissue.
Hepatocytes are responsible for various metabolic functions, while fibroblasts are a type of connective tissue cell involved in maintaining the ECM. Co-culturing these two cell types enabled the team to investigate the interactions between them as well as the role of the ECM in cell simulation.
The researchers observed a significant improvement in cellular functions when co-culturing cells on the SNF scaffold. The unique characteristics of SNF made it an ideal support material for studying cell–cell interactions via paracrine signaling.
While the high porosity of the novel scaffold facilitated the infiltration of cells in a 3D culture environment, its high permeability enabled efficient exchange of signaling molecules (or soluble factors) among different kinds of cells.
“In light of our findings, the novel technology holds immense potential for novel applications such as drug screening, tissue engineering, and regenerative medicine,” concludes Prof. Otsuka.
More information:
Shohei Ishikawa et al, Three‐dimensional co‐culture model employing silica nonwoven fabrics to enhance cell‐to‐cell communication of paracrine signaling between hepatocytes and fibroblasts, Biotechnology and Bioengineering (2023). DOI: 10.1002/bit.28425
Provided by
Tokyo University of Science
Citation:
Novel silica nonwoven fabric scaffold enhances understanding of cell-to-cell interactions (2024, August 22)
retrieved 22 August 2024
from https://phys.org/news/2024-08-silica-nonwoven-fabric-scaffold-cell.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.